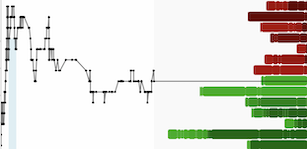
STOCKHOLM, Oct. 25, 2023 /PRNewswire/ -- BioArctic AB's (publ) (Nasdaq Stockholm: BIOA B) partner Eisai today presented new data on the investigational subcutaneous formulation of LEQEMBI showing that it clears 14% more amyloid plaques as measured by PET compared to intravenous infusion (IV), with pharmacokinetics (AUC) 11% higher and similar ARIA rates. An additional analysis of the phase 3 Clarity AD data for LEQEMBI in the PET low tau population revealed that 76% of patients in the low tau population showed no decline and 60% showed clinical improvement at 18 months of treatment.
The data was presented in the Late Breaking Symposium 4 "Lecanemab for Early Alzheimer's Disease: Long-Term Outcomes, Predictive Biomarkers and Novel Subcutaneous Administration" at the 16th annual Clinical Trials on Alzheimer's Disease (CTAD) conference held in Boston, Massachusetts, United States and virtually October 24-27, 2023. Eisai aims to submit a LEQEMBI subcutaneous formulation Biologics License Application (BLA) with the U.S. Food and Drug Administration by March 31, 2024.
- Subcutaneous formulation interim data; safety and effects on brain amyloid
- Weekly subcutaneous (SC) administration showed 14% greater amyloid plaque removal than biweekly IV administration as suggested in a preliminary analysis using amyloid PET at 6 months of treatment.
- The SC substudy, evaluating the SC formulation in an open-label extension (OLE) of the Clarity AD study[1], included 72 patients who received LEQEMBI for the first time as the SC formulation, and 322 patients who received intravenous (IV) LEQEMBI in the Clarity AD Core study followed by SC administration in this substudy. Reduction from baseline of amyloid in the brain by amyloid PET at 6 months in the newly treated SC patients by centiloid reduction was -40.3 ± 2.27 in SC administration compared to -35.4 ± 1.14 in IV administration.
- SC pharmacokinetics (AUC) higher than IV by 11%
- Weekly SC administration of LEQEMBI shows AUC 11% higher than the biweekly IV formulation. The 90% CI for drug exposure for SC vs. IV was within the predefined bioequivalence limits of 80 to 125%. These data could allow Eisai to select a SC dose that achieves AUC that are comparable to the IV dose.
- Lower systemic injection reaction rates with SC as compared to IV
- Systemic injection/infusion reactions are uncommon and mild with SC administration, and in particular have not been observed in patients who received LEQEMBI for the first time as the SC formulation. There was a low rate of local injection site reactions (8.1%) in SC treated patients overall. Most were mild or moderate in severity consisting of redness, irritation, or swelling. No skin rash or other hypersensitivity reactions were reported.
- ARIA rates of IV formulation in Clarity AD core study consistent with rates in first-time LEQEMBI patients entering the SC substudy in Clarity AD OLE
- The incidence of ARIA-E with SC was similar to the IV. The incidences of ARIA-E, ARIA-H and ARIA-H alone (ARIA-H without ARIA-E) with IV in the Clarity AD core study (n=898) were 12.6%, 17.3% and 8.9%, respectively. In newly treated patients in the SC substudy of the Clarity AD OLE (n=72), the incidences of ARIA-E, ARIA-H and ARIA-H alone were 16.7%, 22.2% and 8.3%, respectively. However, due to the sample size of newly treated patients in the SC substudy, no exact comparison can be made.
- Based on Phase II and III clinical studies, Cmax (maximum exposure) was the strongest predictor of ARIA-E incidence following IV administration. In the SC substudy, the steady-state exposure (AUCss) appears to be a better predictor of ARIA-E rates in the SC due to a relatively stable exposure profile.
- Latest data from tau PET longitudinal substudy, including a post-hoc analysis of the low and intermediate + high-tau subpopulations in the Clarity AD 18-month core study
- 76% of patients showed no decline and 60% showed clinical improvement at 18 months in low-tau / earlier stage early AD population.
- The Clarity AD study included an optional tau PET substudy and used the tau PET probe MK6240[2] to identify patients with a low accumulation of tau in the brain, which represents the earlier stage of early AD.
- The low-tau subpopulation, which is in the earlier stages of early AD, is thought to show slow disease progression. In the low-tau subpopulation (n = 70 on lecanemab, n = 71 on placebo), 76% of the LEQEMBI group showed no deterioration and 60% showed clinical improvement after 18 months of treatment in the primary endpoint, Clinical Dementia Rating - Sum of Boxes (CDR-SB), compared with 55% and 28% of the placebo group, respectively.
- Importantly, in this low-tau subgroup, LEQEMBI treatment also showed consistent clinical response across multiple endpoints.[3] In the population studied with tau PET, LEQEMBI treatment favored cognition and function in the earlier stage of early AD.
- The efficacy results of the tau PET substudy in the Clarity AD study, were consistent with overall results of the Clarity AD study.
- Tau PET substudy showed LEQEMBI slows development of tau tangles in early AD; Tau spread in the brain is a hallmark of disease progression.
- In the Clarity AD tau PET substudy, LEQEMBI treatment slowed the buildup of tau proteins in the temporal lobe, where tau accumulation was observed in the earlier stage of early AD. In the tau PET substudy, LEQEMBI suppressed the accumulation of tau in the medial temporal brain region in low-tau subpopulations, and in a broader range of brain regions in the intermediate and higher accumulation groups. This suggests that LEQEMBI treatment may have different effects on brain regions indexed by tau depending on the stage of the disease.
- Efficacy results from LEQEMBI Clarity AD open-label extension study
- LEQEMBI Patients Continued to Show Benefit At 24 Months of Treatment
- In the 18-month core study of Clarity AD, there was a statistically significant difference in global cognition and function as measured by CDR-SB between the LEQEMBI and placebo groups. The separation in CDR-SB between the group that continued to receive LEQEMBI (early start group) and the group who switched from placebo to LEQEMBI (delayed start group) was maintained during the 6-month OLE following the core study. This indicates that similar disease trajectories for both early and delayed start groups can be attained with LEQEMBI administration.
- The blood biomarker results (plasma Aβ42/40 ratio, ptau181, GFAP and NfL) showed improvement also after delayed initiation of treatment with LEQEMBI. These results suggest improvement of AD pathology underlying LEQEMBI treatment effects on clinical outcomes.
- The mechanism of LEQEMBI treatment in early AD
- LEQEMBI has a unique dual acting that binds more selectively to highly toxic forms of amyloid beta (Aβ) (protofibrils[4]) in addition to rapidly clearing plaque, and continues to support neuronal function by removing protofibrils that can cause neuronal injury and death after plaque has been cleared, offering patients the opportunity for continued benefit.
Eisai is hosting a live webcast of the scientific session featuring the LEQEMBI presentations, which can be viewed on the investors section of the Eisai Co., Ltd. website. The content will be available on demand afterward.
Eisai serves as the lead of LEQEMBI development and regulatory submissions globally with both Eisai and Biogen co-commercializing and co-promoting the product and Eisai having final decision-making authority. BioArctic has the right to commercialize lecanemab in the Nordic region and currently Eisai and BioArctic are preparing for a joint commercialization in the region.
This release discusses investigational uses of an agent in development and is not intended to convey conclusions about efficacy or safety. There is no guarantee that such investigational agents will successfully complete clinical development or gain health authority approval.
Please see full U.S. Prescribing Information for LEQEMBI, including Boxed WARNING.
The information was released for public disclosure, through the agency of the contact persons below, on October 25, 2023, at 11.25 p.m. CET.
For further information, please contact:
Oskar Bosson, VP Communications and IR
E-mail: oskar.bosson@bioarctic.se
Phone: +46 70 410 71 80
Jiang Millington, Director Corporate Communication and Social Media
E-mail: jiang.millington@bioarctic.se
Phone: +46 79 33 99 166
About lecanemab (generic name, U.S. and Japan brand name: LEQEMBI®)
Lecanemab is the result of a strategic research alliance between BioArctic and Eisai. Lecanemab is a humanized immunoglobulin gamma 1 (IgG1) monoclonal antibody directed against aggregated soluble (protofibril) and insoluble forms of amyloid-beta (Aβ). In the U.S., LEQEMBI was granted traditional approval by the US Food and Drug Administration (FDA) on July 6, 2023. LEQEMBI is an amyloid beta-directed antibody indicated as a disease-modifying treatment for Alzheimer's disease (AD) in the US. Treatment with LEQEMBI should be initiated in patients with mild cognitive impairment or mild dementia stage of disease, the population in which treatment was initiated in clinical trials. In Japan, Eisai received approval from the Ministry of Health, Labour and Welfare (MHLW) on September 25, 2023, to manufacture and market lecanemab as a treatment for slowing progression of MCI and mild dementia due to AD.
Please see full U.S. Prescribing Information, including Boxed WARNING.
Eisai has also submitted applications for approval of lecanemab in EU, China, Canada, Great Britain, Australia, Switzerland, South Korea and Israel. In China and Israel, the applications have been designated for priority review, and in Great Britain, lecanemab has been designated for the Innovative Licensing and Access Pathway (ILAP), which aims to reduce the time to market for innovative medicines.
Eisai has completed a lecanemab subcutaneous bioavailability study, and subcutaneous dosing is currently being evaluated in the Clarity AD (Study 301) open-label extension (OLE) study. A maintenance dosing regimen has been evaluated as part of the Phase 2b study (Study 201).
Since July 2020 Eisai's Phase 3 clinical study (AHEAD 3-45) for individuals with preclinical AD, meaning they are clinically normal and have intermediate or elevated levels of amyloid in their brains, is ongoing. AHEAD 3-45 is conducted as a public-private partnership between the Alzheimer's Clinical Trial Consortium that provides the infrastructure for academic clinical trials in AD and related dementias in the U.S, funded by the National Institute on Aging, part of the National Institutes of Health and Eisai.
Since January 2022, the Tau NexGen clinical study for Dominantly Inherited AD (DIAD), that is conducted by Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU), led by Washington University School of Medicine in St. Louis, is ongoing and includes lecanemab as the backbone anti-amyloid therapy.
About the collaboration between BioArctic and Eisai
Since 2005, BioArctic has a long-term collaboration with Eisai regarding the development and commercialization of drugs for the treatment of Alzheimer's disease. The most important agreements are the Development and Commercialization Agreement for the lecanemab antibody, which was signed 2007, and the Development and Commercialization agreement for the antibody LEQEMBI back-up for Alzheimer's disease, which was signed 2015. In 2014, Eisai and Biogen entered into a joint development and commercialization agreement for lecanemab. Eisai is responsible for the clinical development, application for market approval and commercialization of the products for Alzheimer's disease. BioArctic has right to commercialize lecanemab in the Nordic under certain conditions and is currently preparing for commercialization in the Nordics together with Eisai. BioArctic has no development costs for lecanemab in Alzheimer's disease and is entitled to payments in connection with regulatory approvals, and sales milestones as well as royalties on global sales.
About BioArctic AB
BioArctic AB (publ) is a Swedish research-based biopharma company focusing on disease-modifying treatments for neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease and ALS. BioArctic focuses on innovative treatments in areas with high unmet medical needs. The company was founded in 2003 based on innovative research from Uppsala University, Sweden. Collaborations with universities are of great importance to the company together with its strategically important global partner Eisai in Alzheimer disease. The project portfolio is a combination of fully funded projects run in partnership with global pharmaceutical companies and innovative in-house projects with significant market and out-licensing potential. BioArctic's Class B share is listed on Nasdaq Stockholm Large Cap (ticker: BIOA B). For more information about BioArctic, please visit www.bioarctic.com.
[1] Phase 3 Clarity AD study is a placebo-controlled, double-blind, parallel-group, randomized study to evaluate the efficacy and safety of LEQEMBI 10 mg/kg bi-weekly for 18 months in 1,795 people living with early AD (core study). An open-label extension study (OLE) is being conducted after the core study. Subcutaneous dosing is currently being evaluated in the Clarity AD OLE.
[2] Using the MK6240 tau PET probe, tau accumulation in the brain was defined as low tau accumulation group (MK6240 cutoff value <1.06, 141 subjects), intermediate accumulation group (MK6240 cutoff value between 1.06 and 2.91, 191 subjects), and high accumulation group (MK6240 cutoff value >2.91, 10 subjects).
[3] Multiple endpoints: CDR-SB, a numeric scale used to quantify the severity of symptoms of dementia; ADAS-Cog14, common cognitive assessment instrument used in AD clinical trials all over the world; and ADCS MCI-ADL, a scale to assess the parties' activities of daily living.
[4] One of the AD pathological features is the accumulation of clusters (plaques) of amyloid beta (Aβ) in the brain. The formation of these plaques is the result of a continuous process by which individual Aβ proteins join together, latching onto each other, one at a time, like adding links to a chain. In the early part of this process these small chains of Aβ are soluble and are toxic to the nerves within the brain. The most toxic of the soluble chains is called a protofibril. Protofibrils are believed to contribute to the brain injury that occurs with AD and are considered to be the most toxic form of Aβ, having a primary role in the cognitive decline associated with this progressive, debilitating condition. Protofibrils cause injury to neurons in the brain, which in turn, can negatively impact cognitive function via multiple mechanisms, not only increasing the development of insoluble Aβ plaques but also increasing direct damage to brain cell membranes and the connections that transmit signals between nerve cells or nerve cells and other cells. It is believed the reduction of protofibrils may prevent the progression of AD by reducing damage to neurons in the brain and cognitive dysfunction.
The following files are available for download:
https://mb.cision.com/Main/9978/3862461/2386751.pdf New data from LEQEMBI® (lecanemab-irmb) phase 3 Clarity AD study and subcutaneous formulation presented at CTAD
![]() View original content:https://www.prnewswire.co.uk/news-releases/new-data-from-leqembi-lecanemab-irmb-phase-3-clarity-ad-study-and-subcutaneous-formulation-presented-at-ctad-301968090.html
View original content:https://www.prnewswire.co.uk/news-releases/new-data-from-leqembi-lecanemab-irmb-phase-3-clarity-ad-study-and-subcutaneous-formulation-presented-at-ctad-301968090.html